Answers
Explanation:
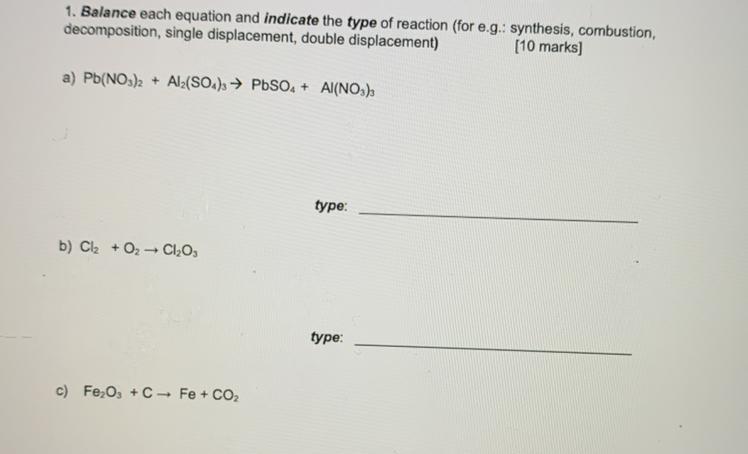
a) 3Pb(NO3)2 + Al2(SO4)3 ---> 3PbSO4 + 2Al(NO3)3
Double displacement
b) 2Cl2 + 3O2 ---> 2Cl2O3
Synthesis
c) 2Fe2O3 + 3C ---> 4Fe + 3CO2
single displacement
Related Questions
The mass number of an atom can be calculated from the
Answers
Mass number (A) =protons (p+) + neutrons(n°)
4. Which of the following process is NOT part of wool extraction?
(a) Shearing (b) Scouring (c) Sorting
(d) Reeling
Answers
Answer:
Reeling is the only process not listed.
The correct answer is option D: Reeling.
Wool is obtained from sheep. The wool so obtained is processed according to the following flow chart;
Shearing → Scouring → Sorting → Dyeing → Straightening, Rolling and Combing
Shearing is the removal of the fleece and thin skin of the sheep. Scouring is the process of washing the hair to remove grease, dust, and dirt. Sorting is the process of differentiating the fibers.
Hence, reeling is not a process in wool extraction.
https://brainly.com/question/9968125
______ is the process of change from a liquid to a gas at temperatures below the boiling
point.
Answers
Answer:
evaporation is the process
Answer:
EvaporationExplanation:
Evaporation is the process of becoming a vapor. The process of extracting moisture as by heat.
Evaporation is the process of change from a liquid to a gas at temperatures below the boiling point.
Therefore, the final answer is evaporation.
describe and compare the trends in hardness for group 1 and group 2
elements?
Answers
Answer:
The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
The hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
What are the group 1 and 2 elements?The group 1 elements are alkali metals and group 2 elements are alkaline earth metals. Group 1 contain 7 elements. Group 2 has 6 elements.
The difference between, group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
Thus, the hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
Learn more about group 1 and 2 elements
https://brainly.com/question/867686
#SPJ2
PLEASE HELP EMERGENCY ‼️‼️ needed ASAP
Answers
Answer:
the photo is kind of blurry i cant really see it, sorry
Explanation:
A tau lepton decays into an electron, an electron antineutrino and a tau neutrino. Write out this reaction in symbolic (equation) form and show that charge and lepton number is conserved. 2. What is the total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons
Answers
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
The total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons is 12 quarks.
What is the meaning of lepton?Any of a family of particles (such as electrons, muons, and neutrinos) that have spin quantum number ¹/₂ and that experience no strong forces.
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
Charge on the left side = -1
Charge on the right side = -1+0+0 = -1
As the charge on the electron antineutrino, [tex]{\bar{\nu }}[/tex] e and the tau neutrino, [tex]\nu\tau[/tex] are zero each.
Thus, charge on left side = charge on right side
Or, the equation is in accordance with charge conservation.
Lapton number of left side = 1
Laptop number of right side = 1-1+1 = 1
This is because, lapton number of the particles involved are,
Tau lapton, [tex]\tau[/tex]-1 = 1,
Electron, e-1 = 1,
Electron antineutrino, [tex]{\bar{\nu }}[/tex]e = -1,
Tau neutrino, [tex]\nu\tau[/tex]= 1,
Thus, lapton number of left side = lapton number of right side
So, the equation obeys lapton number conservation.
2.
The total number quarks in a helium nucleus which is composed of 2 protons (positively charged particle) and 2 neutrons (uncharged particle) is:
Since there 3 quarks in each nucleon, 4 nucleons would have a total of 12 quarks.
Just simply multiply 3 quarks by the number of nucleons:
So, 3 (quarks) x 4 (nucleons) = 12 quarks
Therefore, there are 12 quarks in 4 nucleons.
Learn more about lepton decays here:
https://brainly.com/question/12044097
#SPJ1
what mass of oxygen reacted with the 8.40 grams of c
Answers
For 2-methylbutane, the AH° of vaporization is 25.22 kJ/mol and the
AS° of vaporization is 84.48 J/mol K. At 1.00 atm and 201 K, what is
the AG° of vaporization for 2-methylbutane, in kJ/mol?
Answers
Answer:
ΔG° = ΔH° - TΔS°
ΔG° = 25.22-(201 )(0.08448)
ΔG° = 8.24 kJ/mol
The standard free energy is obtained as 8.2 kJ/mol.
We know that; ΔG° = ΔH° - TΔS° where;
ΔG° = standard free energy
ΔH° = standard enthalpy
T = temperature
ΔS° = standard entropy
Hence substituting the values;
ΔG° =25.22 kJ/mol - (201 K * 84.48 J/mol K)
ΔG° = 25.22 * 10^3 J/mol - 16980.5 J/mol
ΔG° = 8.2 kJ/mol
Learn more about free energy: https://brainly.com/question/1301963
to the nearest gram, what is the mass of of one spoonfull of sugar? g
Answers
Answer:
the mass of one spoonful of sugar to nearest gram is 10g
1. What is the difference between a synthetic resource and a Natural Resource? This is technology.and I need help pls help!!!!!!!!!!!!!
Answers
Answer:
Natural materials are those that are found in nature and have not been made by humans. By comparison, synthetic materials are man-made and cannot be found in nature. Synthetic products are usually created in laboratories by mixing different chemicals, or prepared compounds and substances made in a laboratory.
Explanation:
Natural materials are those that are found in nature and have not been made by humans. By comparison, synthetic materials are man-made and cannot be found in nature. Synthetic products are usually created in laboratories by mixing different chemicals, or prepared compounds and substances made in a laboratory
which is radioactive decay
Answers
Answer:
Explanation:all
Calculate the Ka of a 0.35M weak acid with a pH of 4.2.
Answers
Answer:
Ka = 1.14x10⁻⁸
Explanation:
First we calculate [H⁺] from the pH:
pH = -log[H⁺][H⁺] = [tex]10^{-pH}[/tex][H⁺] = 6.31x10⁻⁵ MFor a monoprotic weak acid, the molar concentration of H⁺ of a solution can be expressed as:
[H⁺] = √(C*Ka)Where C is the molar concentration of the weak acid solution.
6.31x10⁻⁵ M = [tex]\sqrt{0.35M*Ka}[/tex]1.14x10⁻⁸ = KaWhy doesnt the gas on a gas giant escape into space, as it has on mercury?
Answers
Answer:
When surface gravity is ore than the escape velocity gases can not escape from the planet.
Explanation:
Mercury is near Sun and the solar wind is also blowing out the atmosphere.
The gas on a gas giant escape into space, as it does on mercury because mercury is closest to sun.
What is a gas giant?A gas giant is defined as a giant planet which is composed mainly of elements of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranus and Neptune are really a distinct class of giant planets, being composed mainly of heavier volatile substances.For this reason, Uranus and Neptune are now often classified in the separate category of ice giants.
Jupiter and Saturn consist mostly of elements of hydrogen and helium, with heavier elements making up between 3 and 13 percent of their mass. They are thought to consist of an outer layer of compressed molecular hydrogen surrounding a layer of liquid metallic hydrogen, with probably a molten rocky core inside.
Learn more about gas giant,here:
https://brainly.com/question/9401838
#SPJ2
Which base(s) is weaker than ammonia?
hydroxylamine, methylamine, and pyridine
pyridine only
hydroxylamine and methylamine
hydroxylamine and pyridine
Answers
Answer:
Hydroxylamine and pyridine
Explanation:
Just did it
What major component of water affects most its properties?
A. its abundance on Earth
B. its different states
C. polarity
D. its expansion when freezing
Answers
Answer:
C. polarity
Explanation:
Water is polar that is why it is a universal solvent.
What is a empirical formula
Answers
Answer:
The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound. It is determined using data from experiments and therefore empirical.
For example, the molecular formula of glucose is C 6H 12O 6 but the empirical formula is CH 2O.
Please mark as brainliest if answer is right
Have a great day, be safe and healthy
Thank u
XD
Answer:
a chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule CH2O is the empirical formula for glucose.
Which of these is a good conductor?
Plastic
Rubber
Wood
Metal
Answers
A molecule with 14 total electrons and 12 total protons
Answers
Answer:
Explanation:
Tenochtitlan was located on a swampy island in Lake Texcoco in what is today south central Mexico. The Aztecs were able to settle there because no one else wanted the land. At first, it wasn't a great place to start a city, but soon the Aztecs built up islands where they could grow crops. The water also worked as a natural defense against attacks from other cities.
Read more at: https://www.ducksters.com/history/aztec_empire/tenochtitlan.php
This text is Copyright © Ducksters. Do not use without permission.
The charge on a molecule with 14 electrons and 12 protons = -2
Although your question is vague a general answer is provided above
When the number of protons and the number of electrons in a molecule are equal to each other, the charge on the molecule will be neutral, this is because Electrons are negatively charged while protons are positively charged.
Therefore a molecule with 14 electrons and 12 protons will have a charge of
= -14 + 12 = - 2
learn more : https://brainly.com/question/18169295
Calculate the ionization constant for the following acids or bases from the ionization constant of its conjugate base or conjugate acid: Keep your answer to 2 significant figures (CH3)3NH+
Answers
Answer:
7.41 × 10⁻⁵
Explanation:
Let's consider the basic dissociation reaction of trimethylamine (CH₃)N).
(CH₃)N + H₂O = (CH₃)NH⁺ + OH⁻
According to Brönsted-Lowry, in this reaction (CH₃)N is a base and (CH₃)NH⁺ is its conjugate acid. The pKb for (CH₃)N is 9.87. We can calculate the pKa of (CH₃)NH⁺ using the following expression.
pKa + pKb = 14
pKa = 14 - pKb = 14 - 9.87 = 4.13
Then, we can calculate the acid dissociation constant for (CH₃)NH⁺ using the following expression.
pKa = -log Ka
Ka = antilog - pKa = antilog -4.13 = 7.41 × 10⁻⁵
Can anyone please help?
Answers
Answer:
Earth
Explanation:
Earth is unique in the fact that we have an oxygen-rich atmosphere
What is the name of Na
Answers
mdmsjdkskdkskdjxjxjjz
In the compound Na3PO4, which two atoms are involved in the formation of a covalent bond?
Answers
Answer:
P-O.
Explanation:
Hello there!
In this case, according to the definition of the covalent bond, which involves valence electrons sharing, occurring specially between nonmetals, we infer that the P-O bond is the most likely to be covalent, because the P-Na bond is not present and the O-Na bond is ionic.
Regards!
At a temperature of 408K, which gad will have the highest velocity?
Answers
Answer:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Explanation:
Kinetic Energy is directly related to temperature; the higher the temperature the higher the kinetic energy. Kinetic energy is also equal to 12m⋅v2, so if we want a high velocity we want high temp and low mass. So let's list out approximate masses:
m(H2)≈2
m(NO2)≈46
m(Ne)≈20
So we have NO2 at 339 K, the lowest temperature out of the mix, and the highest mass out of the mix, so this is moving the slowest.
In contrast, we have H2 at 425 K, the highest temperature out of the mix, and the lowest mass out of the mix, so this is moving the fastest.
Now we have Ne and H2 at 371 K, since they are at the same temperature they have the same kinetic energy. But H2 is lighter than Ne so it must be faster. To quantify this mathematically, let's assume (this is wrong but just as an assumption for an example) KE at 371 K is 100:
100=12⋅m⋅v2
200=m⋅v2
√200m=v
So H2 is about v=10 and Ne is about v=√10≈3
So the order to recap is:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Hope that makes it clearer!
two differences between weather and climate
subject-science
Answers
Answer:
weather- rain, hail, snow, sunny, dry, etc.
climate- warm, cold, humid, dry, etc.
Explanation:
The difference if weather and climate is that climate is the way the temperature is, whether it's warm, cold, humid, dry. While weather is the state of the atmosphere at a place and time as regards heat, dryness, sunshine, wind, rain, etc.
Which food is the most complete source of amino acids?
A. Bread
B. Fruit
C. Butter
D. Meat
Answers
Answer:
D.Meat
Explanation:
Meat-Most animals proteins are considered complete and high-quality.This includes red meat, poultry,and dairy products.
Amino acids are the molecules that make the protein and are essential nutrients. Meat is the best source that will have amino acids. Thus, option D is correct.
What are amino acids and proteins?Amino acids are the building blocks that make the macromolecule, a protein required for functioning, strength, and activities of the body. Amino acid-containing foods like eggs, meat, and soybeans are rich in proteins.
Amino acids and proteins are essential for body growth and repair that can be gained from poultry sources like eggs, meat, fish, cheese, quinoa, beans, etc. Bread, fruits, and butter are rich in carbohydrates.
Therefore, option D. meat is rich in amino acids.
Learn more about proteins here:
https://brainly.com/question/13161832
#SPJ2
Commercial soaps are mixtures of ionic compounds typically made up of monatomic cations, such as Na and K , and organic polyatomic anions derived from fatty acids. These negatively charged molecular ions are characterized by the presence of hydrocarbon chains which are 12 to 18 carbon atoms long. How hard (solid, insoluble) or soft (liquid, soluble) a soap is depends on the nature of the anions and cations present in the system. Analyze how each of the following factors may affect the hardness or softness of soaps:
1. The nature of the cations. For example, Na* vs Li* vs K.
2. The length of the hydrocarbon chain. For example, 12 carbons (laureate lon), 14 carbons (myristate lon), or 18 carbons (stearate lon).
Answers
Answer:
Following are the solution to the given question:
Explanation:
For question 1:
The sodium soap containing Na+ is strong whereas the softer or liquids were potassium soap.It's hard to use lithium soap.These Na+, K+, and Li+ ions act as the hydrophilic center.Calcium and Magnesium ions could be substituted by hard water with increasing hydrophilicity.For question 2:
The hydrophobicity of its carbon chain increases but one appears weaker with only an increased length.Therefore, the laureate is hard, while the stearate is soft.What is the percent yield if the actual yield of
NH3 is 70 grams
Answers
pls help me with is question.
Answers
Answer:
25cm³ = 0.025L of H2So4
Molarity of H2SO4 = 1moldm-³
Recall ... 1dm-³ = 1L
So the Molarity can also be 1mol/L
Mole = Molarity x volume in L
Mole of H2SO4 = 1mol/L x 0.025L
=0.025Moles of Sulphuric acid reacted.
From the equation of reaction
1mole of H2SO4 reacts to produce 1mole of Copper sulphate crystal
Since their Mole ratio is 1:1
It means that Since 0.025mole of H2SO4 reacted.... 0.025mole of CuSO4.5H2O would be produced
Nice.. OK
So we know the moles of CuSO4.5H2O produced
We can get the Mass
Recall
From
Mole=Mass/Molar Mass
Mass = Mole x Molar Mass
Molar Mass of CuSO4.5H2O = 64 + 32 + 16x4+ 5(2+16)
Mm= 250g/mol
Mass = 0.025mol x 250g/mol
= 6.25g of CuSO4.5H2O crystals Would be PRODUCED.
PLEASE PLEASE HELP!!!!
Approximately what mass of potassium nitrate will eventually precipitate from a supersaturated solution containing 19 grams of the solute in 28 grams of water at 40C°?
Answers
A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution.
Increased temperature usually increases the solubility of solids in liquids.
For example, the solubility of glucose at 25 °C is 91 g/100 mL of water. The solubility at 50 °C is 244 g/100 mL of water.
If we add 100 g of glucose to 100 mL water at 25 °C, 91 g dissolve. Nine grams of solid remain on the bottom. We have a saturated solution.
Hope it helps u
Plz mrk me brainlest
Calculate the kinetic energy of a mole of oxygen gas molecules that have a speed
of 10.0 m/s.
Answers
Answer:
1600J
Explanation:
1 mole of oxygen gas (O2) has a mass of 32g i.e. the molar mass = 32g/mol
Kinetic energy (K.E) = ½ × m × v²
Where;
m = mass (g)
v = speed or velocity (m/s)
From the information given in this question;
m = 32g
V = 10m/s²
K.E = ½ × 32 × 10²
K.E = 16 × 100
K.E = 1600J