The candle is lit and dilute ethanoic acid is poured down the inside of the beaker. As the acid reacts with the baking soda, bubbles of CO2 gas form. After a few seconds
the air in the beaker is replaced by 0.20 liter of CO2 gas, causing candle flame to go out. The density of CO2 gas is 1.8 grams per liter at room temperature.
Choose the correct structural formula for the acid that was poured into the beaker.
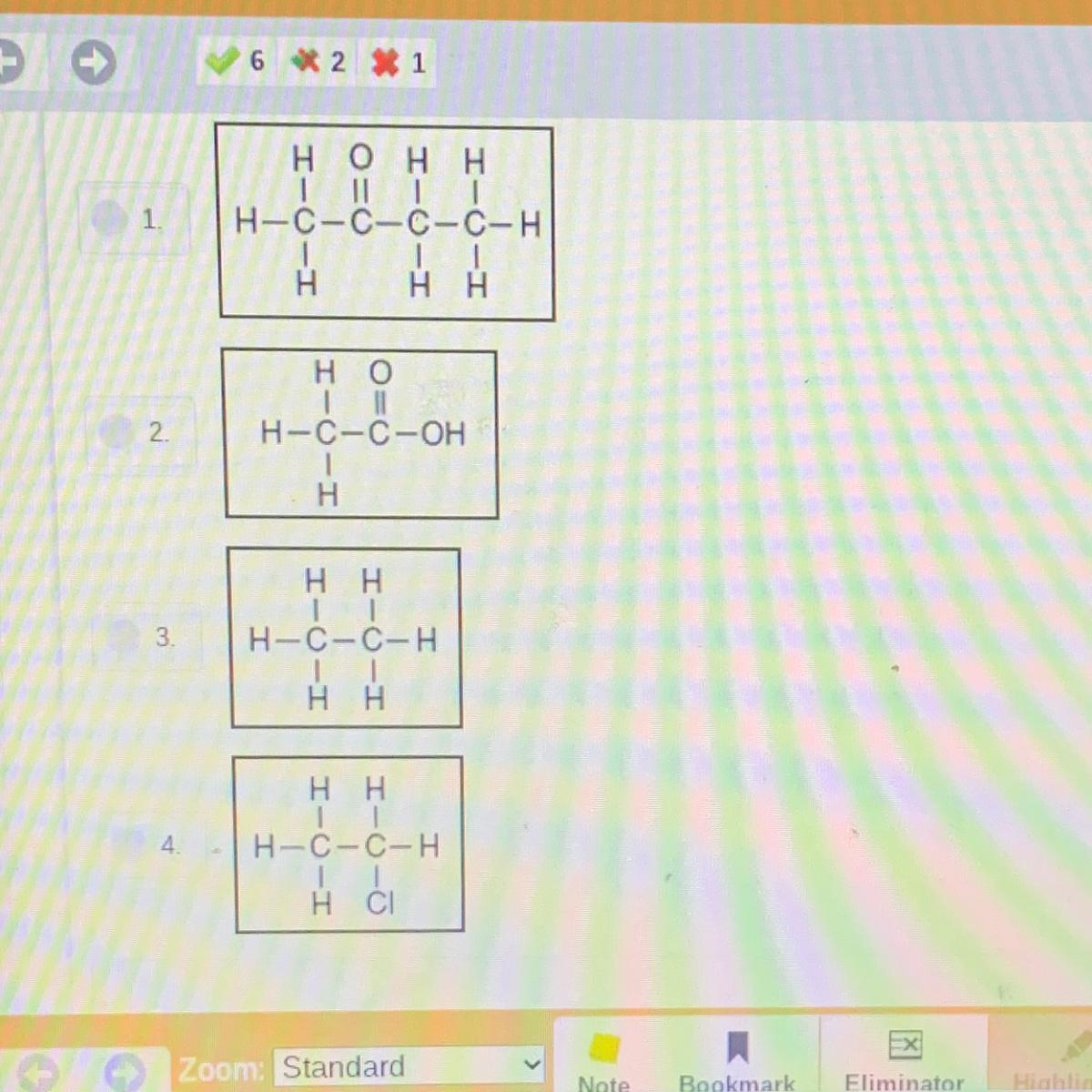
Answers
Answer:
It’s answer #2
Explanation:
I only know this because i just got it wrong
The correct structural formula for the acid that is poured into the beaker is CH₃COOH.
What is acetic acid?Acetic acid is also called ethanoic acid. It is form as a byproduct of fermentation. It forms vinegar. Vinegar is 4 to 6% acetic acid with water.
It is widely used in homes as cleaning agent, in cooking, washing etc.
Thus, the correct option is 2, CH₃COOH.
Learn more about acetic acid
https://brainly.com/question/15202177
#SPJ2
Related Questions
In a nuclear equation:??
Answers
In an effort to maintain homeostasis, the organ systems of the human body work to keep the body's internal water level constant. Which of the following statements describe how the human body responds when its internal water level drops too low?
A. The skeletal system produces more circulating blood cells that insecure the body’s water intake
B. The excretory system signals the kidneys to retain more water and produce more concentrated urine to decrease water loss.
C. The nervous system signals the muscles to constrict , holding more water in the digestive track and decreasing water loss .
D.The excretory system signals the kidneys to release more water into the bladder to increase water loss .
Answers
How many moles of nitrogen are required to produce 13.5 g of NH 3?
Answers
Answer:
number of moles of (N) = 0.794 moles
Explanation:
From the given information:
no of moles of nitrogen (N) = (unknown)???
mass of nitrogen = 13.5 g
molar mass of NH3 = 14 +( 1 × 3) = 17 g/mol
To calculate the no of moles of N, we have:
number of moles of (N) = mass of N/molar mass
number of moles of (N) = 13.5 g/17 g/mol
number of moles of (N) = 0.794 moles
how many moles of Li2SO4 molecules are in 12.71g?
Answers
Answer:
₰₮₤₳V₤
Explanation:
First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.
For NaCl, the molar mass is 58.44 g/mol. Now we can use the rearranged equation. Mass (g) = No. Moles (mol) x Molar Mass (g/mol) = 1 x 58.44. = 58.44 g.
. Explain why some desert animals excrete uric acid rather than ammonia.
(2 marks)
Answers
Answer:
AFAIK
Explanation:
uric acid is much less toxic than ammonia, hence bigger concentrations of it are tolerated in the body. This means you can excrete it while excreting very little water - beneficial wherever water's not abundant.
There's a tradeoff though, uric acid requires more energy to synthesize than ammonia, so pretty much all fish, say, excrete ammonia rather than uric acid - it's no problem to dilute ammonia since there's no water shortage.
intermolecular forces between particles are
Answers
Explanation:
Intermolecular forces hold multiple molecules together and determine many of a substance's properties. All of the attractive forces between neutral atoms and molecules are known as van der Waals forces, although they are usually referred to more informally as intermolecular attraction.
Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions ). These forces are weak compared to the intramolecular forces, such as the covalent or ionic bonds between atoms in a molecule.
Question 4 (10 points)
If a sollition has a pOH of 5.2 the [OH-] of the solution is
оа
6x 10-6 M
Ob
6.3 x 10-6 M
Oc
1.58 x 10-5M
Od
2x10-5M
Answers
Answer:
Explanation:
Which of the following involves a change in chemical properties
Answers
Answer: A chemical change occurs when the substance's composition is changed. When bonds are broken and new ones are formed a chemical change occurs.
4.3 x 10^2 - 7.0 x 10^2 in scientific notation
Answers
ibigay ang kahulugan nito ayun sa paliliwanag
konduktor piloto drayber makinista
Answers
Answer:
konduktor
- ang konduktor ay maaaring isang konduktor nga koryente, train o isang taong namumuno sa isang orkestra o isang grupo ng mga mang-aawit.
piloto
-isang tao na tagalipad ng ereplano
drayber
- isang tao na nagmamaniho ng sasakyan
makinista
-ay isang tao na nagpapakilos o nagpapagana ng makina
please give me brainliest.
When water is boiling in a pot, heat energy is being transferred throughout the water by which type of heat transfer?
O Convection
O Conduction
O Radiation
Answers
Answer:
it would be Convection.
For a chemical reaction, the activation energy for the forward reaction is +181 kJ and the activation energy for the backward reaction is +62 kJ. What is the overall energy change for the forward reaction?
Answers
Given :
For a chemical reaction, the activation energy for the forward reaction is +181 kJ and the activation energy for the backward reaction is +62 kJ.
To Find :
The overall energy change for the forward reaction.
Solution :
The overall energy change for the forward reaction is :
[tex]\Delta E_f = E_f - E_b\\\\\Delta E_f = 181 - 62 \ Kj\\\\\Delta E_f = 119 \ Kj[/tex]
Therefore, the overall energy change for the forward reaction is 119 Kj.
In this experiment, you will need to prepare 250.0 mL of 0.100 M KCl(aq) solution. Determine the mass in grams of potassium chloride required to prepare this solution. Type your numerical answer (no units) in the box below. Even though the number of significant figures is limited to three by the inputs, please report your answer to four significant figures.
Answers
Answer: The mass in grams of potassium chloride required to prepare this solution is 1.862 grams
Explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
[tex]Molarity=\frac{n\times 1000}{V_s}[/tex]
where,
n = moles of solute
[tex]V_s[/tex] = volume of solution in ml
moles of [tex]KCl[/tex] = [tex]\frac{\text {given mass}}{\text {Molar mass}}=\frac{xg}{74.5g/mol}[/tex]
Now put all the given values in the formula of molality, we get
[tex]0.100=\frac{x\times 1000}{74.5g/mol\times 250.0ml}[/tex]
[tex]x=1.862g[/tex]
Therefore, the mass in grams of potassium chloride required to prepare this solution is 1.862 grams
What type of compound is a salt
Answers
Answer: Salt is an ionic compound.
Explanation:
A compound formed by transfer of electrons from one atom to another is called an ionic compound.
For example, chemical formula of salt is NaCl (also called table salt).
Salt is formed when sodium (atomic no. 11) donates its one valence electron to chlorine (atomic no. 17). As a result, sodium ion and chlorine ion chemically combine together and form the compound NaCl.
Thus, we can conclude that salt is an ionic compound.
What determines how atoms will bond?
Answers
Answer:
Explanation:
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms.
Jonathan raised 60 goats, then entered into a series of business transactions. He traded all the goats for sheep at an exchange rate of 5 goats for 7 sheep. Next, he exchanged all the sheep for hogs at a rate of 4 sheep for 2 hogs. How many hogs did he get?
Answers
Answer:
just awser
eeww
Explanation:
what is the ph of a 8.4x10^-6 M H+ solution?
Answers
pH=-log8.4x10^-6
How/what do I answer this?--> "heat of the chemical reactions"
like this is the question :/
Answers
Answer:
The heat of reaction is the energy that is released or absorbed when chemicals are transformed in a chemical reaction. It describes the change of the energy content when reactants are converted into products.
Explanation:
Yeah, that would confuse me a bit but then you read it and then you will get that answer above! Have a great rest of your day!
Colligative properties of solutions include all of the following except: a. an increase in the osmotic pressure of a solution upon the addition of more solute b. elevation of the boiling point of a solution upon addition of a solute to a solvent c. an increase of reaction rate with increase in temperature d. depression of the freezing pont of a solution upon addition of a solute to a solvent e. depression of vapor pressure upon addition of a solute to a solvent
Answers
Answer:
Option C, an increase of reaction rate with increase in temperature
Explanation:
Colligative properties are as follows
a) Decrease of vapor pressure
b) Increase of boiling point
c) Reduction of freezing point
d) Increase of osmotic pressure
There is no impact on reaction rate and hence it is not a colligative property.
Thus, option c is the right choice
An increase of reaction rate with increase in temperature isn't an example
of colligative properties of solutions
Colligative properties of solutions depend on the ratio of the number of
solutes to that of the solvent(concentration) and not on the nature of the
substances involved.
Examples of colligative properties include vapor pressure lowering, boiling
point elevation, freezing point depression, and osmotic pressure. Increase of
reaction rate with increase in temperature is therefore not an example of
colligative properties of solutions.
Read more on https://brainly.com/question/24260365
What molecule represents this structure
A)NH4
B)NH3
C)NH4+
D)NH3+
Answers
Explanation:
As I think Option C is correct i.e. NH4+.
How many grams of water can be heated from 15.0 oC to 55oC using 4250.0 J.
Answers
Answer:
25.42 grams
Explanation:
The specific heat of water is 4.18 J/g •°C, so that means that it takes 4.18 joules of energy to raise one gram of water 1 °C.
Change in temp = 55 - 15 = 40°C
So we can calculate how much energy it would take to raise the temp of one gram of water by one degree using the following
(4.18 J/g•°C) * (40°C) * (1 gram) = 167.2 J
So if it takes 167.2 J of energy to raise on gram of water 40°C, we now we can calculate the amount of grams of water that can heated with 4250 J of energy
(4250 J) ÷ (167.2 J) = 25.42 grams of water can be heated from 15°C to 55°C
What are the components of DNA?
A. ribose sugar, cytosine, guanine, adenine, thymine, and phosphate group
B. ribose sugar, cytosine, guanine, adenine, uracil, and phosphate group
C. deoxyribose sugar, cytosine, guanine, adenine, thymine, and phosphate group
D. deoxyribose sugar, cytosine, guanine, adenine, uracil, and phosphate group
Answers
Answer:
C
Explanation:
A-T G-C
Answer:
C
Explanation:
EDGE 2022
If you have a solution that is 15 percent by mass of KCl in benzene, what is the new boiling point?
Answers
Answer:
https://www.chegg.com/homework-help/questions-and-answers/1-10-points-solution-15-percent-mass-kcl-benzene-new-boiling-point--901-c-b-921-c-c-821-c--q63751186
Explanation: Thats your answer
True or false: If a nuclear chain reaction is uncontrolled, it produces a lot of energy all at once. This is
what happens in an atomic bomb.
Answers
Answer: False, I think...
Explanation:
Answer:
True
Explanation:
It is uncontrolled
Can someone please help me with this question.
Answers
Answer:
A-Sphalerite + Dioxygen+Zinc Oxide+Sulfur Trioxide
B-Sodium+Magnesium+Sodium iodide
Explanation:
Heyyy love good luck i may be wrong on letter B so if it is thats my bad but A should be correct :)
Calculate the maximum work available from 50.0 g of aluminum in the following cell when the cell potential is 1.15 V. Al(s) |Al3+(aq) || H+(aq) | O2(g) |Pt. Note that O2 is reduced to H2O.
Answers
Answer:
W = 615.91 kJ
Explanation:
We need to use the following expression:
W = ΔG° * mol (1)
But in order to determine the ΔG° we need the following expression:
ΔG° = -n * F * E° (2)
Where F is a constant and is 96,500 J/V mol
n is the number of transferred electrons in the reaction. As we are passing from Al to Al³⁺ we can say that the number of electrons are 3.
Finally to get the moles, we need the the atomic weight of aluminum which is 26.98 g/mol, so the moles:
moles = m/MM (3)
Let's calculate the moles of aluminum:
moles = 50 / 26.98 = 1.85 moles of aluminum
Now let's calculate the gibbs energy using (2):
ΔG° = -3 * 96,500 * 1.15
ΔG° = -332,925 J or simply -332.925 kJ/mol
Finally, using (1) we can determine the work done:
W = 332.925 * 1.85
W = 615.91 kJHope this helps
The maximum work available from 50.0 g of aluminum in the following cell when the cell potential is 1.15 V will be 615.91 kJ
What is cell potential ?The cell potential, Ecell, is the measure of the potential difference between two half cells in an electrochemical cell.
Expression for work done ;
W = ΔG° x mol (1)
But, to determine the ΔG°
ΔG° = -n x F x E° (2)
Where,
F is a constant and is 96,500 J/V moln is the number of transferred electrons in the reaction.(As there is variation from Al to Al³⁺ the change in number of electrons are 3)
To get the moles,
moles = m/MM (3)
moles = 50 / 26.98 = 1.85 moles of aluminum
Now,
ΔG° = -3 x 96,500 x 1.15
ΔG° = -332,925 J
= -332.925 kJ/mol
Now, using (1) we can determine the work done:
W = 332.925 x 1.85
W = 615.91 kJ
Hence, The maximum work available from 50.0 g of aluminum in the following cell when the cell potential is 1.15 V will be 615.91 kJ
Learn more about work done here ;
https://brainly.com/question/26622905
#SPJ5
which molecule is butene
Answers
Answer:
Option C is the answer
Butene, also called Butylene, 4 isomeric compound belonging to the series of olefinic hydrocarbons. The chemical formula is C4H8, option c is correct.
What are the 4 isomers of butene?Butene, also called Butylene, four isomeric Combinations belong to the series of olefinic hydrocarbons. The chemical formula is C4H8.
The isomeric forms are 1-butene, cis-2-butene, trans-2-butene, and isobutylene.
Thus, option "C" is correct. the chemical formula is C4H8.
To learn more about Butene click here:
https://brainly.com/question/13186233
A gas has a volume of 6.00 liters at a temperature of 300 K and a pressure of 1.00 atm what is the volume of the gas in liters at a temperature of 600 K and a pressure of 3.00 atm
Answers
Answer:
V₂ = 4.00 L
Explanation:
Given that:
Volume (v1) = 6.00 L
Temperature (T1) = 300 K
Pressure (P1) = 1.00 atm
VOlume (V2) = unknown???
Temperature (T2) = 600 K
Pressure (P2) = 3.00 atm
Using combined gas law equation:
[tex]\dfrac{P_1V_1}{T_1}= \dfrac{P_2V_2}{T_2}[/tex]
[tex]\dfrac{1 \times 6}{300} = \dfrac{3 \times V_2}{600}[/tex]
[tex]\dfrac{1}{50} = \dfrac{1 \times V_2}{200}[/tex]
200 = 50V₂
V₂ = 200/50
V₂ = 4.00 L
Write the balanced half-reaction that occurs at the anode in a lead-acid (storage) battery during discharge. Phases are optional. anode half-reaction: Write the balanced half-reaction that occurs at the cathode in a lead-acid (storage) battery during discharge. Phases are optional. cathode half-reaction: Write the balanced overall cell reaction that occurs in the lead-acid (storage) battery during discharge. Phases are optional. overall cell reaction:
Answers
Answer: Anode: [tex]Pb+SO_4^{2-}\rightarrow PbSO_4+2e^-[/tex]
Cathode: [tex]PbO_2+4H^++SO_4^{2-}+2e^-\rightarrow PbSO_4+2H_2O[/tex]
Overall cell reaction : [tex]Pb+2SO_4^{2-}+PbO_2+4H^+\rightarrow 2PbSO_4+2H_2O[/tex]
Explanation:
Lead storage battery is a secondary cell used in automobiles and invertors. The anode is made up of lead and undergoes oxidation during discharging and cathode is made up of lead oxide and acts as cathode during discharging. The electrolyte used is dilute .
Lead storage battery acts as electrochemical cell while discharging.
Discharging reaction for Anode:
Discharging reaction for Cathode: [tex]PbO_2+4H^++SO_4^{2-}+2e^-\rightarrow PbSO_4+2H_2O[/tex]
Overall cell reaction : [tex]Pb+2SO_4^{2-}+PbO_2+4H^+\rightarrow 2PbSO_4+2H_2O[/tex]
A lead storage battery is an energy storage device. At the anode lead and sulfate ion reacts to produce Lead(II) sulfate and release two electrons.
What are anode and cathode?In secondary cells or the recharge-discharge cells during recharge, the positive electrode is the anode, while during discharge cathode is the positive electrode.
The anode (lead) of the cell undergoes an oxidation reaction during the discharge, whereas the lead oxide or the cathode undergoes reduction.
The discharging reaction at the anode of the cell is given as,
[tex]\rm Pb + SO_{4}^{2-} \rightarrow PbSO_{4} + 2e^{-}[/tex]
The discharging reaction at the cathode of the cell is given as,
[tex]\rm PbO_{2} + 4H^{+} + SO_{4}^{2-} + 2 e^{-} \rightarrow PbSO_{4} + 2H_{2}O[/tex]
The overall cell reaction is given as,
[tex]\rm Pb + 2SO_{4}^{2-} + PbO_{2} + 4H^{+} \rightarrow 2PbSO_{4} + 2H_{2}O[/tex]
Therefore, the gain and loss of electrons are represented at the cathode and the anode of the cell.
Learn more about discharge battery here:
https://brainly.com/question/8341588
What is the Molar mass of zinc
Answers
65.38 u (or g)
I just searched it up